AOP-KF® Patented Material

New Bio-clean Material -
AOP-KF® Solid Alkali
Our patented AOP-KF® solid alkali material emits and maintain a constant level of ClO₂ harmless to human and animals. ClO₂ is a necessary condition for in situ regeneration of hydroxyl radicals (·OH). Without heating, ClO₂ causes physical adsorption of water on the surface of a carrier (mesoporous material). In addition to chemical hydrolysis, there is also non-ionized (radical) hydrolysis of chemisorbed water (OH). This phenomenon can be greatly accelerated when a reacting substrate or a chemical catalysis is present. A large amount of active particles (hydroxyl radicals ·OH) are generated. Hydroxyl radicals have high oxidation potential (2.8eV) while also having a high chemical reaction rate constant. As a result, AOP-KF® is able to eliminate pollutants much more effectively compared to other competing technologies.
| Micro-nano green material. Advanced catalytic oxidation material |
| Regenerating concentrated of ·OH, at an oxidation rate of 106-1010 L/(mol·s), 7 magnitudes higher than that of Ozone (O3) |
| Killing bacteria and virus effectively |
| SGS certified 100% non-toxic and harmless to human, plants and animals |
The Race On Formaldehyde Removal
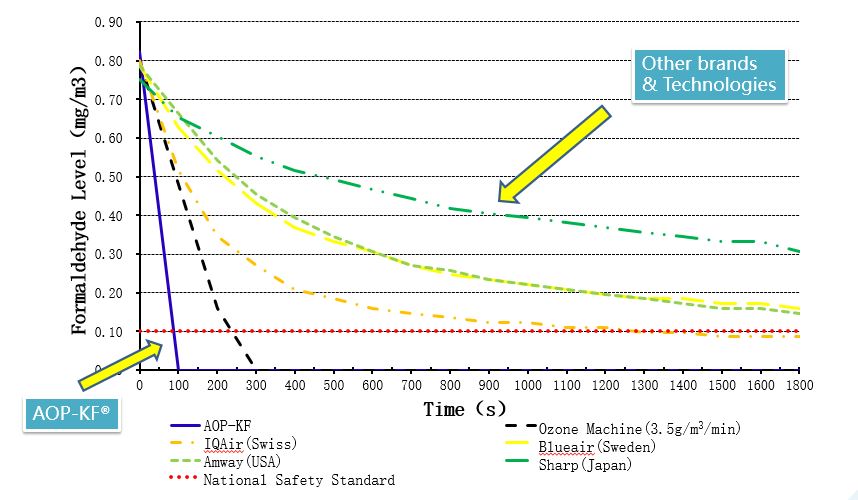
AOP-KF® Superior Removal For Harmful Chemical And Particulate Pollutants
 |
 | |
| Model | YKJ1000F-A01 | YKJ2000F-A01 |
| Particle CADR | 1240.6m³/h | 1998.7m³/h |
| Particle Purification Efficiency | 7.75m³/(w*h) | 6.67m³/(w*h) |
| Formaldehyde CADR | 239.6m³/h | 431.1m³/h |
| Toluene CADR | 58.5m³/h | 123.8m³/h |
The Race On Germs Killing
AOP-KF® Sterilization Rate
| Model YKJ1000F-A01 | ||
| Staphylococcus Aureus | 5min | 95.78% |
| 10min | 99.90% | |
| 15min | 99.99% | |
| Tested by Guangzhou Testing Center of Industrial Microbiology | ||
| Model YKJ2000F-A01 | ||
| Staphylococcus Aureus | 5min | 99.63% |
| 10min | 99.99% | |
| 15min | > 99.99% | |
| Aspergillus Niger | 15min | 99.46% |
| 30min | > 99.96% | |
| Tested by Institute of Geochemistry, Chinese Academy of Sciences | ||
AOP-KF® Virus Removal Rate
| Model YKJ1000F-A01 |
| Phage: Phi x 174 (ATCC 13706-B1) |
| 20 min, > 99.99% |
| (30m3 test chamber) |
| Tested by Guangzhou Testing Center of Industrial Microbiology |
| Model YKJ2000F-A01 |
| A/PR8/34(Influenza A H1N1 Virus) |
| 15 min, > 99.99% |
| (30m3 test chamber) |
| Tested by Testing Center of GIR Medicine Company Limited |
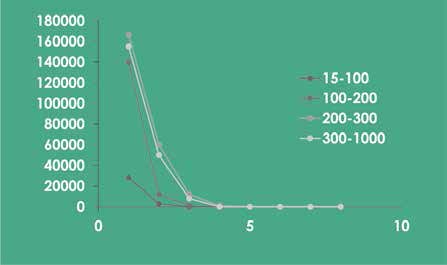
Removal of Fine Particulate Matter (PM1) and Ultrafine Particulate Matter (PM0.1)
| Model YKJ2000F-A01 | ||||
| Time (min) | Diameter (nm) | Initial Concentration (x100个/L) | Final Concentration (x100个/L) | Removal Rate (%) |
| 8 | 15-100 | 28192 | <1 | >99.99 |
| 100-200 | 139694 | <1 | >99.99 | |
| 200-300 | 166469 | <1 | >99.99 | |
| 300-1000 | 155142 | <1 | >99.99 | |
| Tested by Guangzhou Testing Center of Industrial Microbiology and Institute of Geochemistry, Chinese Academy of Sciences | ||||